T. J. Coles: TOTT NEWS
Genetically-modified mRNA lipid nanoparticles were injected into billions of people even while the biodistribution and pharmacokinetic effects remain unknown in humans.
Pfizer’s partner, the German company BioNTech, published an internal 2,237-page report on its animal experiments.
The report parses phrases, saying things like: there was no “systematic” lipid nanoparticle toxicity detected in the rodents. So, there was toxicity.
In addition, the raw data are not included in the report. Their reference numbers are redacted and, under German law, will be kept secret for 15 years before being destroyed.
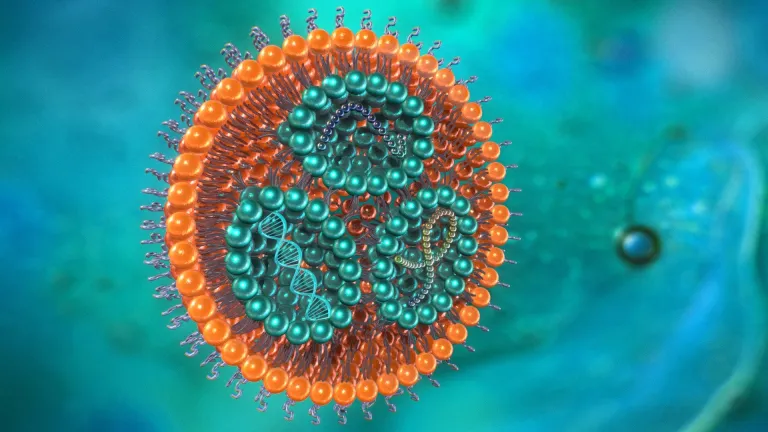
LIPID NANOPARTICLES
Lipids–or fats–naturally constitute cell membranes, regulate cell penetration, store vitamins, aid the production of hormones, and much more.
A nanometre is one-billionth of a metre. Nanoparticles can be organic, like volcanic dust, or human-made, such as carbon tubes. Their dimensions are between one and 100 nanometres.
Since the 1990s, scientists have experimented with modified lipid nanoparticles as a vehicle for novel therapeutics.
The aim is to effectively hide the therapeutics in the lipids, like Trojan horses, so that the body does not reject the therapeutic. Once inside the cell, the therapeutic reproduces and in theory stimulates an immune response.
Messenger RNA (mRNA) mediates between genes and proteins. Antigens are molecules that bind to T-cells and antibodies, and typically trigger an immune response. The Pfizer-BioNTech injectable product is, in theory, an mRNA antigen.
Our bodies are electric. Negatively-charged cell surfaces are called anionic.
mRNA membranes are anionic and thus rejected by cell membranes. The mRNA is thus encased in positively-charged lipid nanoparticles (cationic) that enter the given cell.
POTENTIAL DANGERS
As late as mid-2019, just prior to the pandemic, it was understood that “Lipid nanoparticles (LNPs) tend to accumulate in the liver.”
The study noting this important fact added: “the mechanisms that promote delivery to other cell types within the liver microenvironment are poorly understood.”
Yet, we are supposed to believe that Pfizer and BioNTech mastered this mystery within months to deliver a safe product.
The Pfizer-BioNTech product uses two types of lipids: PEGylated and phospholipids.

PEG is polyethylene glycol. Pfizer, BioNTech, and governments allowed the experimental, genetically-modified mRNA lipid nanoparticle product to be injected into billions of people worldwide despite PEG causing anaphylactic shock–a severe immune response–in people allergic to PEG.

In addition to PEG, cationic lipids (positively-charged) were widely reported in the pre-COVID literature as being cytotoxic, i.e., toxic to cells, as we shall soon see.
But then governments declared a global pandemic and an apparently necessary rush to rollout an alleged vaccine based, in part, on cationic lipids, and suddenly the toxicity was forgotten.
POSITIVE CHARGE, NEGATIVE CONSEQUENCES
Cationic (re)agents are abbreviated (+)NP, as in positively-charged nanoparticles. A study from 2010 said:
“Mice treated with (+)NPs showed increased liver enzyme release and body weight loss compared to mice treated with neutral or negatively charged NPs ((−)NPs), suggesting hepatotoxicity.”
The latter refers to liver toxicity.
Eight years later, the safety of cationic agents was still in question. Lipids are non-viral vectors for gene therapy.
A 2018 study–just a couple of years before the “vaccine” was rolled out–said:

“As effective non-viral vectors …, cationic lipids still have the problem of toxicity, which has become one of the main bottlenecks for their applications.”
Another study from late-‘19, just before the pandemic was announced, said: “cationic reagents are generally cytotoxic.”
BIONTECH’S RATS
The latest document dump contains thousands of pages downplaying the toxicity of lipid nanoparticles, apparently found in the organs of test animals.
The document was approved in September 2020.
BioNTech used 255 Han Wistar rats. Some were injected with failed vaccine candidates, others were injected with what became Comirnaty. Some were injected with a 30 µg dose, others with a 100.
So-called dose exposure is important because, in theory, vaccinologists aim to give the lowest dose with the highest efficacy. But the BioNTech report says from the outset:
“The analysis of dose exposure was conducted under the responsibility of the Sponsor is excluded from this statement.”
So, how do we know what effect the dose exposure had?
PROBLEMS WITH THE REPORT
The first problem is that the report was rewritten at the request of the sponsor, but the report also claims that the rewrites did not make any substantive differences. This suggests a cover-up.
The second problem is that major mistakes were made in the first draft.
This calls into question the accuracy of the second draft:
“…changes for albumin [protein made by the liver] and globulin [immune system-produced protein] levels were incorrectly stated as an increase in albumin and a decrease in globulin plasma levels instead of [vice versa].”
Small activating RNAs are different from mRNAs, yet they interact in ways described as “foggy” just 18 months before the animal trials.
The third problem is that the initial draft confused small activating RNAs with mRNAs.
This is a serious problem if the tests were designed to study the effect of mRNA.
Does this mean that the draft or the actual tests were confused?
The fourth problem was that the vaccine candidates ending in b1 and a1 were mixed up initial tables.
Were the wrong candidates injected into the poor rats or merely reported incorrectly?
DOWNPLAYING TOXICITY
Despite being told that the injection was “safe and effective” for humans, the BioNTech report notes that many experimental rats had adverse reactions. But the language minimises the severity.
Depending on the dose number and potency, “a few” rats experienced “severe oedema” (swelling). Others endured erythema (skin inflammation). Some of the animals’ skins had shed (eschar) to the point where the injection site had to be moved for the second and/or third doses.
Some rats suffered muscle death (necrosis) and fibrosis (damaged tissue).
In the females:
“Inflammation extended into tissues adjacent to the injection site, including mammary tissue, perineural [nerve group] tissue of sciatic nerve, tissue around the femur/knee and to the draining lymph node (iliac).”
What appear to be mRNA and small activating RNA–we don’t know which because the original draft was wrong–were present in the rodents:
“Test item-related microscopic findings at the end of dosing were evident in injection sites and surrounding tissues, increased cellularity of germinal centres [structures in white blood cells] and increased plasma cells in the draining (iliac) lymph nodes, bone marrow, spleen, and liver.”
The report casually mentions “enlarged spleens.”
TOXICITY IN HUMANS
People started getting mRNA injections from December 2020. But a study published as late as mid-2022 said:

“The biodistribution and pharmacokinetics of the mRNA-containing lipid nanoparticles (LNPs) in these vaccines are unknown in humans.”
So, for nearly two years, billions of people had been injected, some multiple times, with a novel product whose distribution in their bodies was unknown.
The study also said:
“We found that vaccine-associated synthetic mRNA persists in systemic circulation for at least 2 weeks.”
A recent article investigating myocarditis in adolescents and young people found elevated spike protein in their blood:
“…markedly elevated levels of full-length spike protein …, unbound by antibodies, were detected in the plasma of individuals with postvaccine myocarditis, whereas no free spike was detected in asymptomatic vaccinated control subjects.”
Another studied chronic hepatitis C virus (HCV) patients:
“In 10 of 108 HCV patient samples, full-length or traces of SARS-CoV-2 spike mRNA vaccine sequences were found in blood up to 28 days after COVID-19 vaccination.”
Necrotising encephalitis (swelling) often results in brain lesions. Vasculitis describes blood vessel inflammation.
In October 2022, a study investigated the death of a Parkinson’s Disease patient who had been injected three times.
The first was with the non-mRNA Oxford-AstraZeneca jab, the second and third were with mRNA products. The patient had no history of having COVID-19.
“[H]istopathological analyses of the brain uncovered previously unsuspected findings, including acute vasculitis (predominantly lymphocytic) as well as multifocal necrotizing encephalitis of unknown etiology with pronounced inflammation including glial [central nervous system cells] and lymphocytic reaction.”
CONCLUSION
Pre-COVID data on lipid nanoparticles (LNPs) suggested that they are toxic to cells and that reducing toxicity is not only difficult but has not been explored in clinical trials.
In addition, it was acknowledged in peer-reviewed medical literature that the distribution of LNPs and mRNA spike protein in humans was not understood.
Despite this, governments approved Pfizer-BioNTech’s injectable product.
The animal trial data suggest that toxicity was downplayed in the published reports.
Even more appalling, the products injected into the animals and the results were mixed up in the first draft, bringing the integrity of the second into serious question. Dose analyses were not even counted.
This had the effect of bringing an experimental product to a captive market under emergency use authorisation, when, in non-pandemic times, such risky products would never have been approved.
TO READ THE PREVIOUS ARTICLES IN THIS SERIES SEE BELOW